Handling Molecular Formulae
Introduction
The molecular formula is the simplest way to characterize a molecular compound. It specifies the actual number of atoms of each element contained in the molecule. A molecular formula is represented by the chemical symbol of each constituent element. If a molecule contains more than one atom for a particular element, the quantity is shown as subscript after the chemical symbol. Otherwise, the number of neutrons (atomic mass) that an atom is composed can differ. This different type of atoms are known as isotopes. The number of nuclei is represented as superscripted prefix previous to the chemical element. Generally it is not added when the isotope that characterizes the element is the most occurrence in nature, e.g., .
Parsing a Molecule To a Molecular Formula
Front a molecule, defined as conjunct of atoms helding together by
chemical bonds, we can simplify it taking only the information about the
atoms. The rcdk package provides a parser to translate
molecules to molecular formlulas, the get.mol2formula
function.
## Loading required package: rcdklibs## Loading required package: rJava
sp <- get.smiles.parser()
molecule <- parse.smiles('N')[[1]]
convert.implicit.to.explicit(molecule)
formula <- get.mol2formula(molecule,charge=0)Note that the above formula object is a
CDKFormula-class. This class contains some attributes that
defines a molecular formula. For example, the mass, the charge, the
isotopes, the character representation of the molecular formula and the
IMolecularFormula
jobjRef object.
The molecular mass, charge and string representation for this formula are given by
formula@mass## [1] 17.02655
formula@charge## [1] 0
formula@string## [1] "H3N"The isotopes for this formula. It is formed from three attributes.
isoto (the symbol expression of the isotope),
number (number of atoms for this isotope) and
mass (exact mass of this isotope).
formula@isotopes## isoto number mass
## [1,] "N" "1" "14.003074"
## [2,] "H" "3" "1.007825032"Depending of the circumstances, you may want to change the charge of the molecular formula.
formula <- set.charge.formula(formula, charge=1)Initializing a Formula from the Symbol Expression
Other way to create a cdkFormula is from the symbol
expression. Thus, setting the characters of the elemental formula, the
function get.formula parses it to an object of
cdkFormula-class.
formula <- get.formula('NH4', charge = 1)
formula## cdkFormula: [H4N]+ , mass = 18.03383 , charge = 1Generating Molecular Formula
Mass spectrometry is an essential and reliable technique to determine
the molecular mass of compounds. Conversely, one can use the measured
mass to identify the compound via its elemental formula. One of the
limitations of the method is the precision and accuracy of the
instrumentation. As a result, rather than specify exact masses, we
specify tolerances or ranges of possible mass, resulting in multiple
candidate formulae for a given mass window. The
generate.formula function returns a list of formulae which
have a given mass (within an error window)
mfSet <- generate.formula(18.03383, window=1,
elements=list(c("C",0,50),c("H",0,50),c("N",0,50)),
validation=FALSE)
mfSet## [[1]]
## cdkFormula: H4N , mass = 18.03437 , charge = 0
##
## [[2]]
## cdkFormula: CH6 , mass = 18.04695 , charge = 0
##
## [[3]]
## cdkFormula: H18 , mass = 18.14085 , charge = 0It is important to know if an elemental formula is valid. The method
isvalid.formula provides this function. Two constraints can
be applied, the nitrogen rule and
the RDBE
rule (Ring Double Bond Equivalent).
formula <- get.formula('NH4', charge = 0)
isvalid.formula(formula,rule=c("nitrogen","RDBE"))## [1] FALSEWe can observe that the ammonium is only valid if it is defined with charge of +1.
formula <- get.formula('NH4', charge = 1)
isvalid.formula(formula,rule=c("nitrogen","RDBE"))## [1] TRUEThe generate.formula method can perform these validation
tests during formula generation by setting validation=TRUE.
However, this can significantly slow down the process of genersating
formulae, especially with larger mass windows. When the default of
FALSE is used, nonsensical formulae may be generated, and
it’s up to the user to filter them out.
In contrast, the generate.formula.iter method employs
the Round Robin formula generation algorithm, which is significantly
faster than the default method. In contrast to
generate/formula, this method returns an iterator which
allows you to process large formula sets without excessive memory
usage.
mit <- generate.formula.iter(100, charge=0, window=0.1,
elements=list(c("C",0,50), c("H",0,50), c("N",0,50)))
hit <- itertools::ihasNext(mit)
while (itertools::hasNext(hit))
print(iterators::nextElem(hit))## [1] "[12]C8[1]H4"
## [1] "[12]C7[1]H2[14]N"
## [1] "[12]C6[14]N2"
## [1] "[12]C4[1]H10[14]N3"
## [1] "[12]C3[1]H8[14]N4"
## [1] "[12]C2[1]H6[14]N5"
## [1] "[12]C[1]H4[14]N6"
## [1] "[1]H2[14]N7"Note that by default, this function will not check for validity of the generated formulae.
Calculating Isotope Pattern
Due to the measurement errors in medium resolution spectrometry, a
given error window can result in a massive number of candidate formulae.
The isotope pattern of ions obtained experimentally can be compared with
the theoretical ones. The best match is reflected as the most probable
elemental formula. rcdk provides the function
get.isotope.pattern which predicts the theoretical isotope
pattern given a formula.
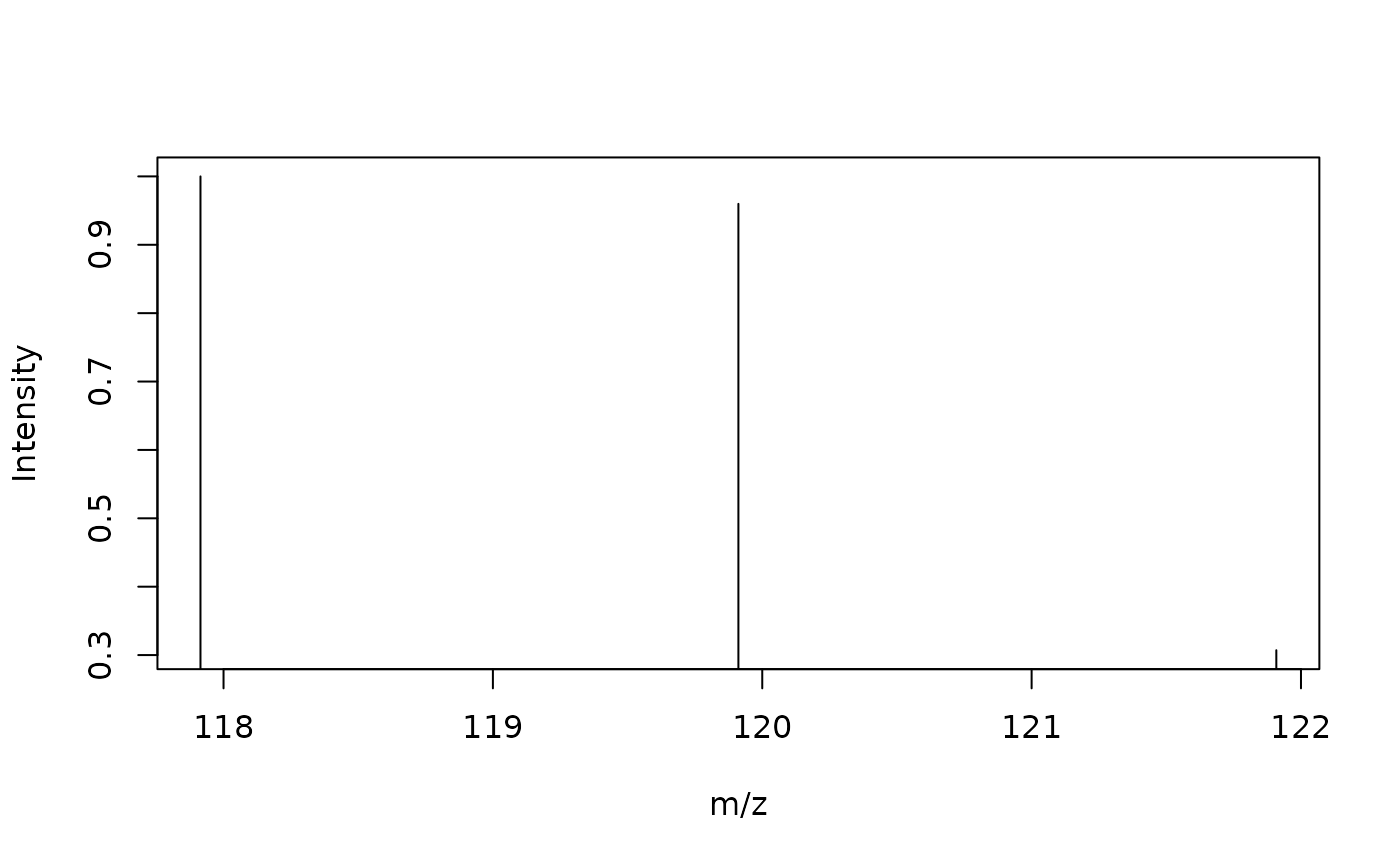
formula <- get.formula('CHCl3', charge = 0)
isotopes <- get.isotopes.pattern(formula,minAbund=0.1)
isotopes## mass abund
## [1,] 117.9144 1.0000000
## [2,] 119.9114 0.9598733
## [3,] 121.9085 0.3071189In this example we generate a formula for a possible compound with a charge () containing the standard elements C, H, and Cl. The isotope pattern can be visually inspected, as shown below.
plot(isotopes, type="h", xlab="m/z", ylab="Intensity")